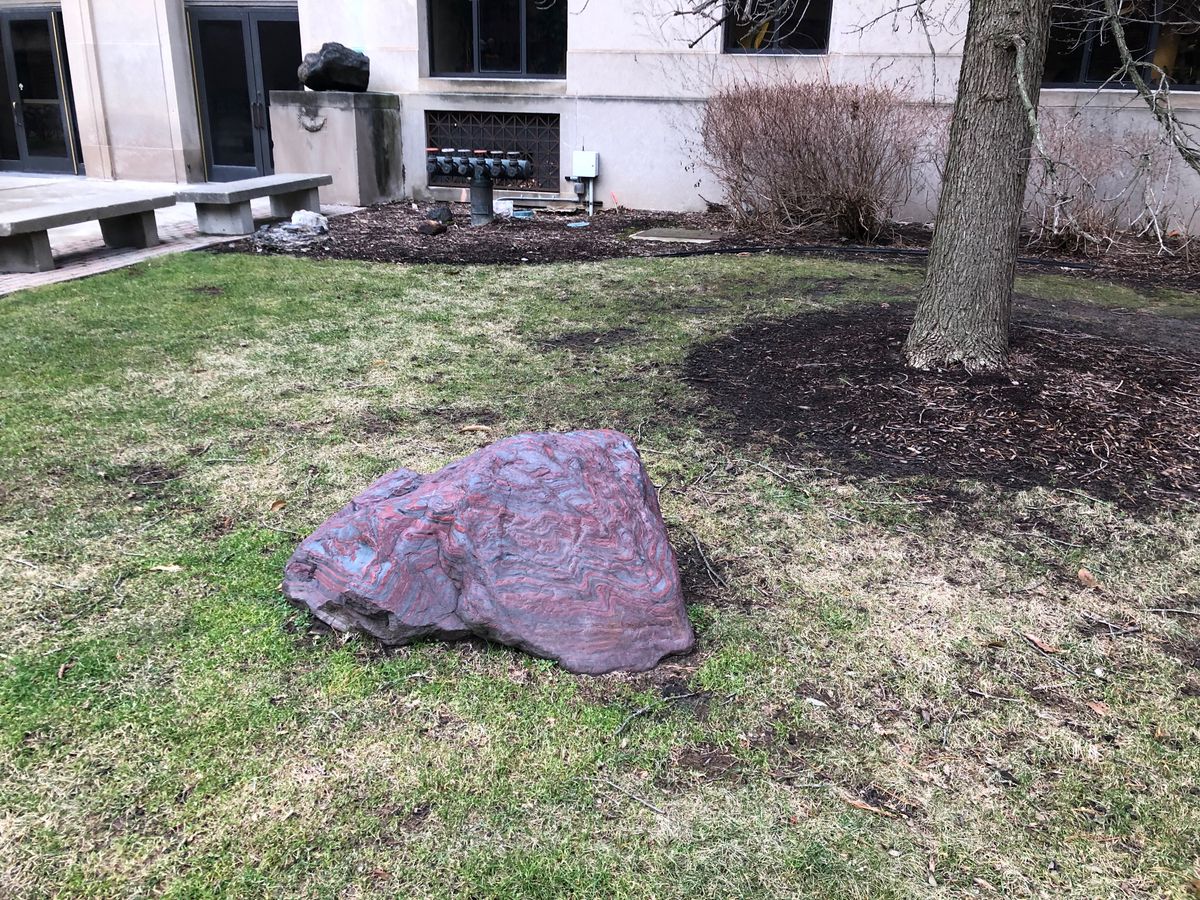About
Three billion years ago, Earth was a very different place than it is today. Even if we could visit, the air itself would have been toxic to modern life as there was no free oxygen in the atmosphere. The lifeforms that did exist were largely bacteria and other microbes. Since we don't have the methods or the means to study this hostile environment up close, scientists use the artifacts from the geological record such as banded iron formations, like this one at the University of Michigan in Ann Arbor, to learn about the ancient earth.
In ancient Earth's oxygen-less environment, most iron eroded out of rocks would have eventually been washed into the ocean. But with the emergence of photosynthetic bacteria, free oxygen started entering the water as a waste product of their metabolic processes. This oxygen reacted with dissolved iron in the water to form iron oxide minerals like magnetite and hematite, which sank to the seafloor.
Some geologists suggest that the characteristic layers of the banded iron formations are a result of the changing seasons. As the number of oxygen-producing organisms fluctuated between seasons, oxygen production rose and fell. When the oxygen was low, bright red layers of oxygen-poor minerals like jasper or chert were laid down over the magnetite and hematite. When their numbers rose again, a new layer of metallic minerals was produced. These seasons may have occurred in an annual cycle or they may have taken hundreds of years, but they repeated over and over they created a distinct type of sedimentary rock that we now know as a banded iron formation.
Banded iron formations stopped forming about 1.8 billion years ago, when Earth’s atmosphere finally became saturated with oxygen and took on a composition more like what we know today. Outcroppings of these formations can be found around the world. One good-sized hunk of this stone is lying in a rock garden on the campus of the University of Michigan outside the 1100 North University Ave. Building. The stone specimen serves as a vivid reminder of the ancient past for students studying Earth's long history, as the building houses several of university departments devoted to geology.
Related Tags
Know Before You Go
The stone is located on the lawn near the west side of the 1100 North University Ave. Building across from the Samuel Trask Dana Building, which houses the School for Environment and Sustainability. The buildings are located on the eastern side of the University of Michigan Diag.
Published
February 12, 2020
Sources
- https://campusinfo.umich.edu/building-search/building/28/1100-north-university-ave-building-central-campus-transit-center
- http://jersey.uoregon.edu/~mstrick/RogueComCollege/RCC_Lectures/Banded_Iron.html
- https://www.amnh.org/exhibitions/permanent/planet-earth/how-has-the-earth-evolved/banded-iron-formation